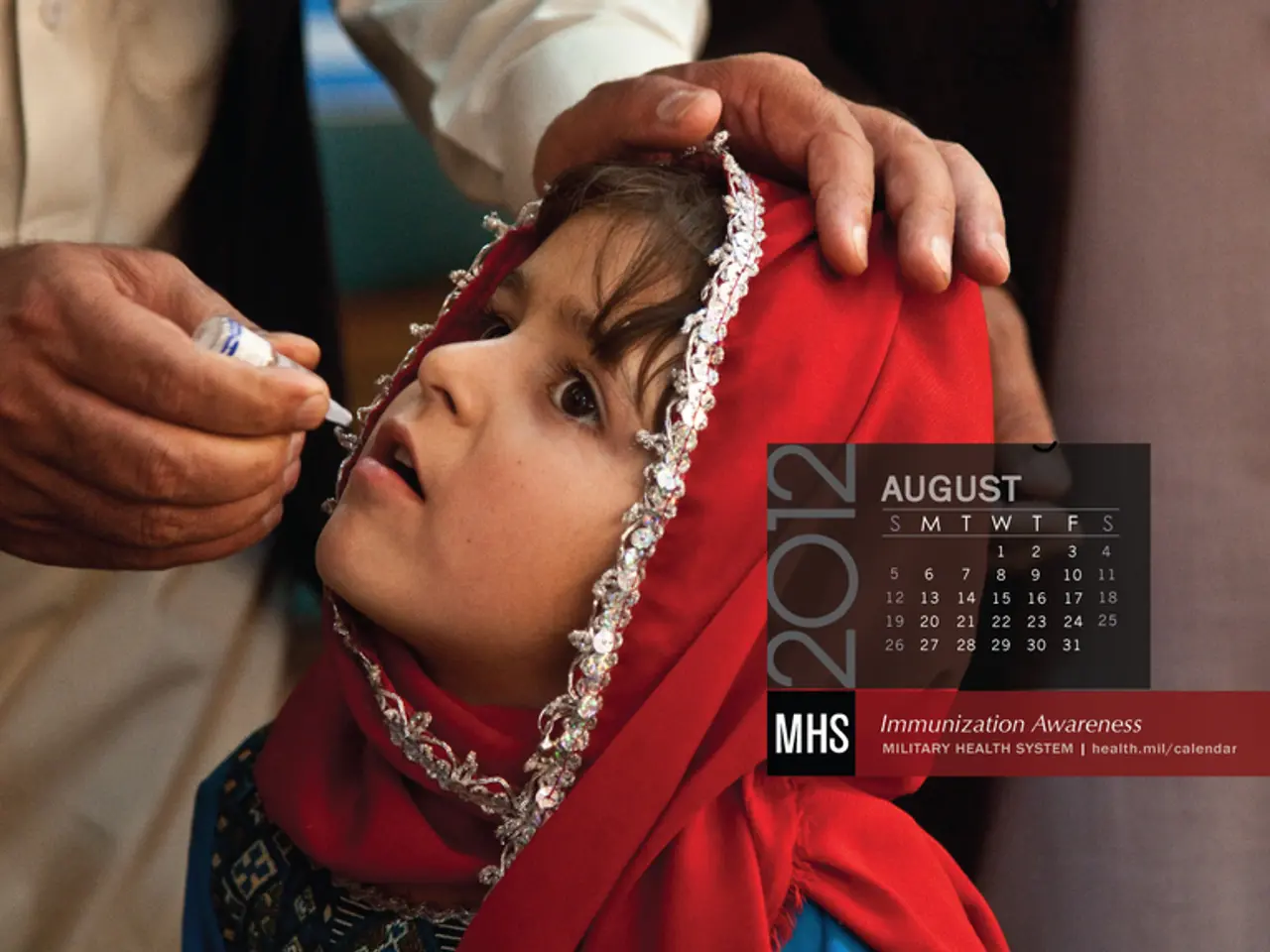
Vaccination of newborns with the hepatitis B vaccine remains crucial, according to medical professionals' assertions.
The medical community and advocacy groups are voicing their concerns as the Advisory Committee on Immunization Practices (ACIP) prepares to vote on a new recommendation regarding the hepatitis B birth dose. The meeting, now composed of 12 members hand-picked by health secretary Robert F Kennedy Jr., has the potential to significantly impact the health of American children.
If ACIP were to reverse the current recommendation for universal hepatitis B vaccination at birth, a majority of the over half a million American kids eligible for shots through the Vaccines for Children program could lose access. This could lead to a rise in hepatitis B cases and associated health risks, as well as potential complications with health insurance coverage.
The hepatitis B vaccine has been shown to be safe and has virtually eliminated hepatitis B among babies in the United States over the past 30 years. In fact, before 1991, hepatitis B shots were only given to infants considered high risk, but this strategy missed many cases. A universal hepatitis B vaccine strategy was adopted to ensure no high-risk infants were missed.
The CDC currently recommends a timely administration of a hepatitis B vaccine to help prevent transmission of the virus from mother to child at birth. This recommendation has been crucial in the fight against hepatitis B, a virus that can lead to chronic infections, liver disease, and even liver cancer.
In 1999, there was a temporary pause in the universal recommendation, in favor of a risk-based recommendation for a brief period that year. This resulted in at least one child in Michigan dying of hepatitis B infection due to improper documentation. Since then, the hepatitis B vaccine has been given within 24 hours after birth, and doctors and chairpersons of the Hepatitis B Foundation argue that this practice is safe, effective, and has nearly eliminated pediatric Hepatitis B in the US.
Doctors and advocates believe that delaying the hepatitis B birth dose may lead to gaps in health insurance coverage, growing health disparities, confusion, and an increase in preventable hepatitis B infections. It's estimated that up to 2.4 million people are living with chronic hepatitis B in the U.S., many asymptomatic and unaware of their diagnosis.
Merck, which makes one of the FDA-approved hepatitis B vaccines, has distributed 330 million doses worldwide since its approval in 1986 and has been closely monitoring the safety of its shot for over 35 years. The hepatitis B vaccine has been shown to be safe, and it's worth noting that in 2023, there were at least 2,214 reports of acute hepatitis B cases in the U.S., which corresponds to an estimated 14,400 acute infections with the virus, after adjusting for unrecognized or underreported infections.
Jade A. Cobern, MD, MPH, a practicing physician and medical fellow of the ABC News Medical Unit, emphasizes the importance of the hepatitis B vaccine. "The hepatitis B vaccine has been a game-changer in the fight against this virus," she says. "Delaying the vaccine could potentially undo the progress we've made in eliminating pediatric hepatitis B in the US."
The ACIP is expected to vote on a new recommendation regarding the hepatitis B birth dose on Thursday. The decision could have significant implications for the health of American children and the fight against hepatitis B.
Read also:
- High recovery rate for over 90% of patients, asserts the head physician of Almaty's 32nd polyclinic, regarding mobile treatment groups.
- Bee colonies in Zirndorf city have been affected by American foulbrood - a designated restriction zone has been established - no immediate threat to local residents.
- Federal Health Care Blueprint for 2026 Revealed by OPM Outlining Key Strategies and Objectives
- Unveiling the Undiscussed Issues of Earbuds: Revealing the Silent Reality